The Earth’s climate is a solar powered system, receiving light energy emitted by the Sun in the form of ultraviolet, visible, and near-infrared radiation. Globally, over the course of the year, the Earth system —land surfaces, oceans, and atmosphere— absorbs an average of about 240 watts of solar power per square meter (one watt is one joule of energy every second).
The absorbed sunlight drives photosynthesis, fuels evaporation, melts snow and ice, and warms the Earth system. The Sun doesn’t heat the Earth evenly. Because the Earth is a sphere, the Sun heats equatorial regions more than polar regions. The atmosphere and ocean work non-stop to even out solar heating imbalances through evaporation of surface water, convection, rainfall, winds, and ocean circulation. This coupled atmosphere and ocean circulation is known as Earth’s heat engine.
Earth’s heat engine moves heat from one part of the surface to another and from the Earth’s surface and lower atmosphere back to space. This flow of incoming and outgoing energy is Earth’s energy budget.
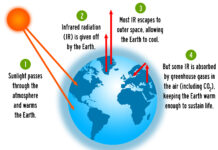
For Earth’s temperature to be stable over long periods of time, incoming energy and outgoing energy have to be equal. This state of balance is called radiative equilibrium. NASA illustration below by Robert Simmon. Astronaut photograph ISS013-E-8948.
About 29 percent of the solar energy that arrives at the top of the atmosphere is reflected back to space by clouds, atmospheric particles, or bright ground surfaces like sea ice and snow. This energy plays no role in Earth’s climate system.
Another 23 percent of the incoming solar energy is absorbed in the atmosphere by water vapor, dust, and ozone, thus warming the atmosphere. The remaining 48 percent of the solar energy passes through the atmosphere and is absorbed by the earth’s surface. Thus, about 71 percent of the total incoming solar energy is absorbed by the Earth system.
The light energy (white arrows) reaching the earth’s surface warms it. The earth’s surface then reflects some of this energy back into the atmosphere as heat (orange arrows), and this creates additional warming of the atmosphere.
Radiatively active gases (i.e., greenhouse gases) such as water vapor, carbon dioxide, ozone, methane, nitrous oxide, and chlorofluorocarbons are fairly transparent to the short wavelengths of solar radiation but efficient at absorbing the longer wavelengths of the infrared radiation (i.e. heat) emitted by the earth and the atmosphere.
Further, much of the heat energy reflected by the earth’s surface into the atmosphere is captured by these gases in the planet’s atmosphere, and they radiate this energy in all directions. Part of this radiation is directed back towards the earth’s surface, thus warming it.[1] Vaclav Smil (2003). The Earth’s Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN 978-0-262-69298-4.
The intensity of downward radiation – that is, the strength of the greenhouse effect – depends on the amount of greenhouse gases that the atmosphere contains. The temperature rises until the intensity of upward radiation from the surface, balances the downward flow of energy.[2] Rebecca, Lindsey (14 January 2009). “Climate and Earth’s Energy Budget : Feature Articles”. earthobservatory.nasa.gov. Retrieved 14 December 2020.
Energy flows between space, the atmosphere, and Earth’s surface, with greenhouse gases in the atmosphere capturing a substantial portion of the heat reflected from the earth’s surface.
Because the Earth’s surface is colder than the Sun, it radiates energy at wavelengths that are much longer than the wavelengths that it absorbs. By their percentage contribution to the greenhouse effect on Earth the major greenhouse gases (GHGs) are[3] Kiehl, J.T.; Trenberth, Kevin E. (February 1997). “Earth’s Annual Global Mean Energy Budget” (PDF). Bulletin of the American Meteorological Society. 78 (2): 197–208. … Continue reading:
– water vapor, 36–70%
– carbon dioxide, 9–26%
– methane, 4–9%
– ozone, 3–7%
Greenhouse gases—including most diatomic gases with two different atoms (such as carbon monoxide, CO) and all gases with three or more atoms—are able to absorb and emit infrared radiation. Though more than 99% of the dry atmosphere is IR transparent (because the main constituents—N2, O2, and Ar—are not able to directly absorb or emit infrared radiation), intermolecular collisions cause the energy absorbed and emitted by the greenhouse gases to be shared with these other, non-IR-active, gases.
The earth’s surface temperature is controlled by long-lived, heat trapping, greenhouse gases, i.e. carbon dioxide – CO2, methane – CH4, nitrous oxide – N2O, and ozone – O3 but excluding water-vapor. Earth’s surface temperature will continue rising as the concentration of these gases in the atmosphere continues to increase.
In addition to the above and since the middle of the 20th century, small amounts of novel man-made gases, mostly chlorine- and fluorine-containing solvents and refrigerants, have also been added to the mix. Because these gases are not condensable at atmospheric temperatures and pressures, the atmosphere can pack in much more of these gases, all leading to a build-up of CO2, CH4, N2O, and O3 in the atmosphere.
Greenhouse Gas Emissions by Type of Gas
Long-lived non-condensable gases that remain semi-permanently in the atmosphere and do not respond physically or chemically to changes in temperature are described as “forcing” climate change. This article from the Carbon Brief, with interactive graphics, shows the relative contributions of different forcings i.e. the various factors that affect the amount of energy that reaches and remains in the Earth’s climate.
Gases, such as water vapor, which respond physically or chemically to changes in temperature are seen as “feedbacks.”
Water vapor[4]“Water vapour: feedback or forcing?”. RealClimate. 6 April 2005. Retrieved 1 May 2006., the most important greenhouse gas, is thought to increase in concentration in response to increased concentrations of the other greenhouse gases as a result of feedback mechanisms in the climate system. If there had been no increase in the amounts of non-condensable greenhouse gases, the amount of water vapor in the atmosphere would not have changed with all other variables remaining the same. The addition of the non-condensable gases causes the temperature to increase and this leads to an increase in water vapor, via evaporation from the earth’s surface, and this further increases the temperature. This is an example of a positive feedback effect.
The combination of these forcing and feedback gases results in the warming of the atmosphere and consequently the planet.
References
| ↑1 | Vaclav Smil (2003). The Earth’s Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN 978-0-262-69298-4. |
|---|---|
| ↑2 | Rebecca, Lindsey (14 January 2009). “Climate and Earth’s Energy Budget : Feature Articles”. earthobservatory.nasa.gov. Retrieved 14 December 2020. |
| ↑3 | Kiehl, J.T.; Trenberth, Kevin E. (February 1997). “Earth’s Annual Global Mean Energy Budget” (PDF). Bulletin of the American Meteorological Society. 78 (2): 197–208. Bibcode:1997BAMS…78..197K. CiteSeerX 10.1.1.168.831. doi:10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2. Archived from the original (PDF) on 30 March 2006. Retrieved 1 May 2006. |
| ↑4 | “Water vapour: feedback or forcing?”. RealClimate. 6 April 2005. Retrieved 1 May 2006. |